Bambu Lab A1 Combo, A1 3D Printer and AMS lite, Support Multi-Color 3D Printing, High Speed & Precision, Full-Auto Calibration & Active Flow Rate Compensation, ≤48 dB Quiet FDM 3D Printers
(as of June 19, 2025 23:45 GMT +00:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)3D Systems has achieved 510(k) clearance from the FDA for their patient-specific VSP PEEK Cranial Implant solution. This innovative technology utilizes additive manufacturing to produce cranial implants with up to 85% less material than traditional machining methods, leading to significant cost savings. The VSP PEEK Cranial Implant is the first FDA-cleared, additively manufactured PEEK implant, offering exceptional performance and biocompatibility for cranioplasty procedures. With several successful surgeries already completed using this solution, the potential for customized PEEK cranial plates to become routine in clinical practice is promising.

Article Title
3D Systems Achieves 510(k) Clearance for PEEK Cranial Implants
Introduction
In a groundbreaking announcement, 3D Systems has received 510(k) clearance from the FDA for their patient-specific VSP PEEK Cranial Implant. This approval signifies a significant advancement in the field of medical technology, allowing for the production of personalized cranial implants using 3D printing technology. The VSP PEEK Cranial Implant solution includes a complete FDA-cleared workflow and offers numerous benefits, such as reduced material usage, cost savings, and faster turnaround times. This article will explore the background information, benefits, successful case studies, expert opinions, and features of the VSP PEEK Cranial Implant, shedding light on the immense potential and impact of this innovative solution in the medical field.
$30 off $400+ Anycubic Products with code AC30OFF
Background Information
FDA approval for 3D printed cranial implant
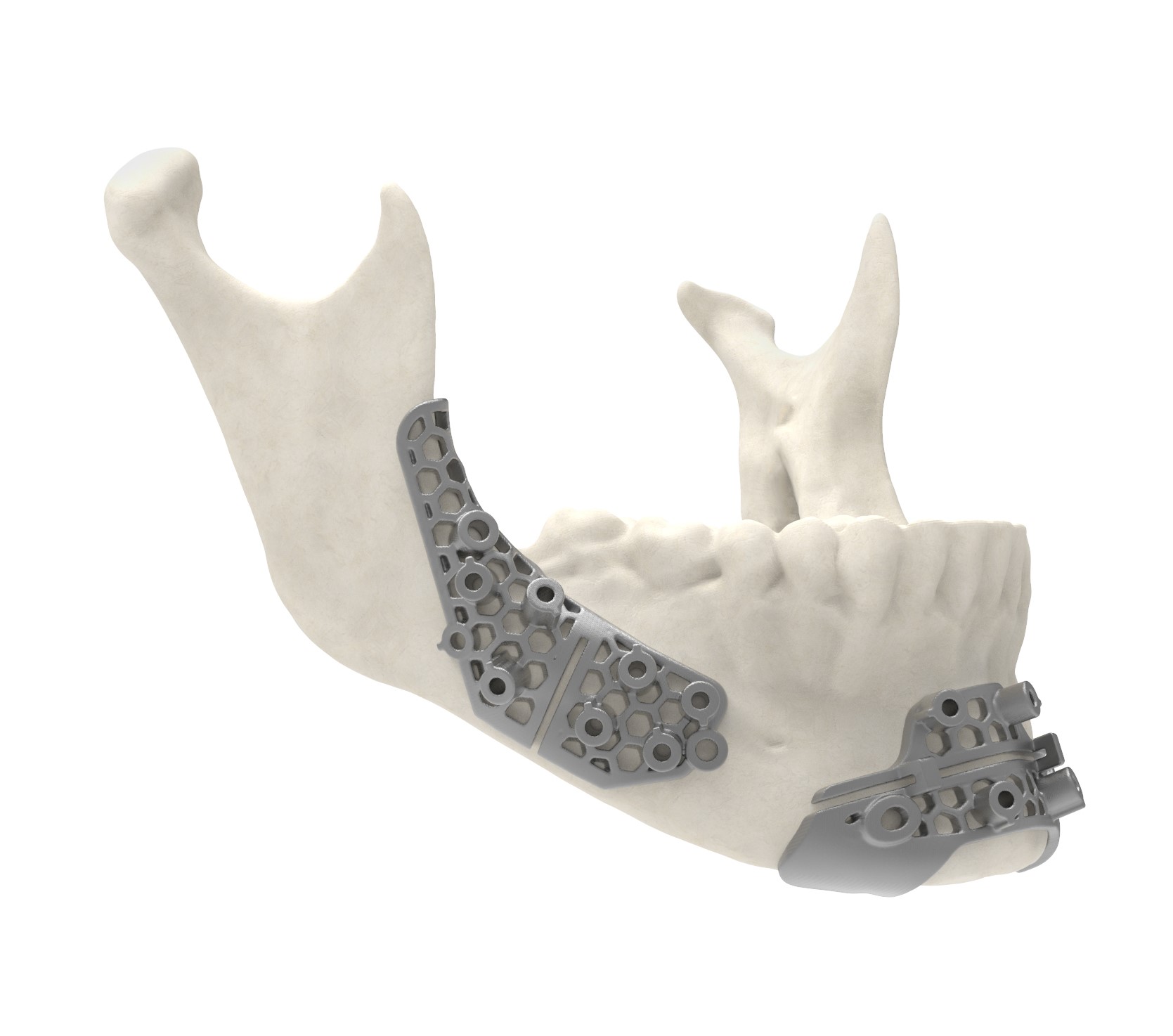
The FDA’s clearance of the VSP (Virtual Surgical Planning) PEEK Cranial Implant marks a significant milestone in the adoption of 3D printing in the healthcare industry. This approval validates the safety and efficacy of using 3D printing technology to produce patient-specific cranial implants. By utilizing additive manufacturing (AM) solutions, 3D Systems has revolutionized the production process, enabling the creation of customized implants with reduced material usage and improved performance.
VSP PEEK Cranial Implant solution
The VSP PEEK Cranial Implant solution offered by 3D Systems encompasses a complete workflow, ensuring seamless integration of 3D printing technology into clinical practice. This solution includes segmentation and 3D modeling software, the 3D Systems EXT 220 MED 3D printer, Evonik VESTAKEEP i4 3DF PEEK (polyetheretherketone) material, and a pre-defined production process. By encompassing every step of the production process, from software modeling to implant creation, the VSP PEEK Cranial Implant solution ensures a streamlined and efficient workflow.
Complete FDA-cleared workflow
One of the key advantages of the VSP PEEK Cranial Implant solution is its complete FDA-cleared workflow. This means that every step of the process, from software modeling to implant production, has met the rigorous standards set by the FDA. By obtaining 510(k) clearance, 3D Systems has demonstrated the safety and efficacy of their technology, giving healthcare professionals confidence in utilizing the VSP PEEK Cranial Implant solution for patient care.
Benefits of AM Solutions
Reduced material usage
The use of 3D printing technology in the production of cranial implants allows for significant reduction in material usage. Traditional machining methods often result in excessive material waste, driving up costs and impacting sustainability. However, with AM solutions, such as the VSP PEEK Cranial Implant, the production process is optimized to utilize only the necessary amount of material, resulting in up to 85% less material usage compared to traditional machining methods.
Cost savings
The reduced material usage inherent in AM solutions also leads to substantial cost savings. Implantable materials, such as PEEK, can be expensive, and minimizing waste during the production process significantly reduces costs. By adopting 3D printing technology, hospitals and healthcare facilities can achieve cost-efficiency without compromising the quality or performance of the implants.
Cleanroom-based architecture
The cleanroom-based architecture of the 3D Systems EXT 220 MED 3D printer ensures a sterile environment for implant production. This feature is crucial for medical applications as it eliminates the risk of contamination during the manufacturing process. The cleanroom environment ensures that the cranial implants produced are of the highest quality, meeting the stringent standards required for medical devices.
Faster turnaround time
Using AM solutions, such as the VSP PEEK Cranial Implant, significantly reduces the turnaround time for producing patient-specific cranial implants. Traditional methods often involve lengthy lead times, from custom tooling to machining and finishing processes. However, with 3D printing technology, the entire process can be streamlined, allowing healthcare professionals to provide faster treatment for patients in need of cranial implants.
Controlled overall cost
Controlled overall cost is another key benefit of adopting AM solutions in the production of cranial implants. The reduction in material usage, cost savings, and faster turnaround time all contribute to a more efficient and cost-effective process. This controlled overall cost not only benefits healthcare facilities but also ensures that patients have access to affordable and personalized medical solutions.
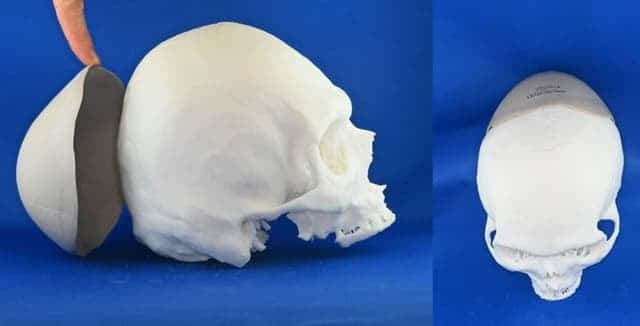
Successful Case Studies
Cranioplasties in Switzerland
The VSP PEEK Cranial Implant solution has already been successfully utilized in Switzerland at University Hospital Basel for nearly 40 cranioplasty procedures. These procedures have provided patients with customized cranial implants, tailored specifically to their unique anatomical requirements. The successful outcomes of these surgeries demonstrate the efficacy and potential of 3D printing technology in improving patient care.
Cranioplasties in Austria
Salzburg University Hospital in Austria has also witnessed the successful integration of the VSP PEEK Cranial Implant solution into routine clinical practice. Surgeons at this hospital have completed numerous surgeries utilizing the patient-specific cranial implants, with excellent results. The ability to customize implants using 3D printing technology has opened up new possibilities for precise and individualized neurosurgery, improving patient outcomes and expanding the capabilities of surgeons.
Cranioplasties in Israel
At Tel-Aviv Sourasky Medical Center in Israel, the VSP PEEK Cranial Implant solution has been instrumental in achieving successful cranioplasties. This innovative solution, combined with 3D Systems’ cutting-edge printing technology and the mechanical properties of PEEK, has pushed the boundaries of what can be achieved in neurosurgery. The integration of 3D printing into routine clinical practice offers significant potential for improving patient care and revolutionizing the field.
Expert Opinions
Dr. Johannes Pöppe on the innovative solution
Dr. Johannes Pöppe, a Senior Attending Surgeon at the Department of Neurosurgery in the University Hospital Salzburg, praises the innovative solution offered by 3D Systems. According to Dr. Pöppe, the combination of 3D Systems’ printing technology, uniquely engineered for sterile environments, and the mechanical properties of PEEK has resulted in a groundbreaking solution for neurosurgery. He believes that the customization potential of PEEK cranial plates is significant in integrating 3D printing into routine clinical practice, and the VSP PEEK Cranial Implant solution is revolutionizing the field of neurosurgery.
Integration of 3D printing into routine clinical practice
The integration of 3D printing into routine clinical practice is a topic of great interest and potential. With the FDA clearance of the VSP PEEK Cranial Implant, the use of 3D printing technology in healthcare is becoming more widespread. The success of the VSP PEEK Cranial Implant solution in Switzerland, Austria, and Israel demonstrates that additive manufacturing has the potential to greatly contribute to patient care and the advancement of neurosurgery.

Features and Advantages of VSP PEEK Cranial Implant
First FDA-cleared PEEK implant for cranioplasty procedures
The VSP PEEK Cranial Implant is the first additively manufactured PEEK implant to receive FDA clearance for cranioplasty procedures. This achievement underscores the effectiveness and safety of 3D printing technology in producing patient-specific medical devices. The FDA clearance serves as a testament to the rigorous testing and validation that the VSP PEEK Cranial Implant has undergone, reassuring healthcare professionals of its quality and reliability.
Mechanical properties similar to human bone
PEEK, the material used in the VSP PEEK Cranial Implant, possesses mechanical properties that closely mirror human bone. This remarkable characteristic ensures that the implants provide the necessary support and integration with the patient’s existing cranial structure. By replicating the mechanical properties of human bone, the VSP PEEK Cranial Implant facilitates optimal healing and reduces the risk of complications.
Excellent biocompatibility and resistance to bodily fluids
Biocompatibility and resistance to bodily fluids are crucial considerations in the development of medical devices. The VSP PEEK Cranial Implant has demonstrated excellent biocompatibility, ensuring that it is well-tolerated by the patient’s body. Furthermore, its resistance to bodily fluids enhances the longevity and durability of the implant, reducing the need for frequent replacements or revisions.
Minimal interference in medical imaging
An essential feature of any cranial implant is its compatibility with medical imaging techniques. The VSP PEEK Cranial Implant exhibits minimal interference in medical imaging, allowing for clear and accurate evaluation of the surgical site and the integrity of the implant. This feature greatly assists healthcare professionals in monitoring the healing process and ensuring the proper integration of the implant with the patient’s cranial structure.
Conclusion
The FDA clearance of the VSP PEEK Cranial Implant marks a significant milestone in the field of 3D printing technology and medical device manufacturing. The innovative solution offered by 3D Systems provides healthcare professionals with a comprehensive workflow that allows for the production of patient-specific cranial implants. The benefits of adopting AM solutions, such as reduced material usage and cost savings, combined with the excellent biocompatibility and mechanical properties of the VSP PEEK Cranial Implant, make it a game-changer in neurosurgery and cranial reconstruction. As more hospitals and healthcare facilities integrate 3D printing into routine clinical practice, the potential for improving patient care and expanding the possibilities of precise and individualized medical solutions grows exponentially. With the VSP PEEK Cranial Implant, 3D Systems has set a new standard for cranial implants, paving the way for the future of personalized medicine.
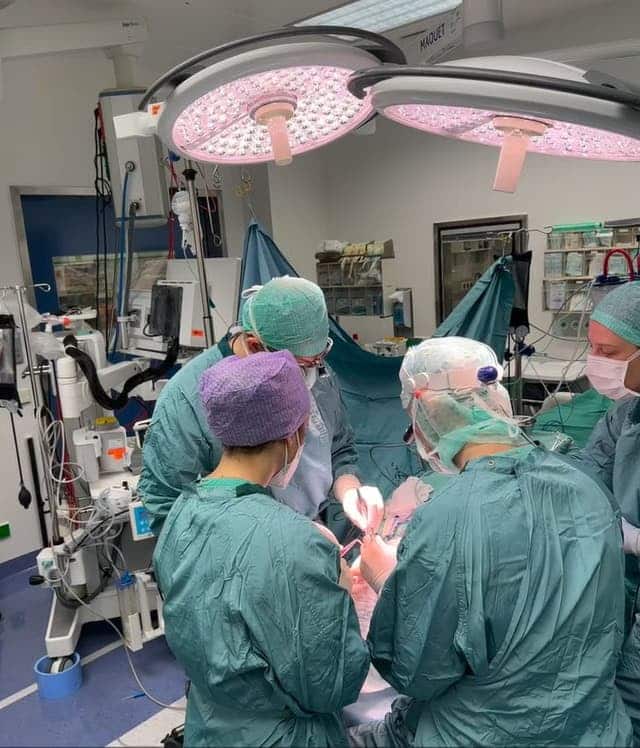
Additional News and Related Content
Anycubic’s new 3D printer and online community launch
In addition to the groundbreaking advancements in cranial implant technology, 3D printing continues to revolutionize various industries. Anycubic, a leading manufacturer of 3D printers, has recently launched a new color-switching 3D printer, a high-speed resin 3D printer, and an online community. These developments highlight the continuous innovation and growth within the 3D printing industry, offering new opportunities and possibilities for professionals and hobbyists alike.
Axtra3D’s focus on practical applications for HPS 3D printing process
Axtra3D, a company specializing in high-performance thermoplastic materials, has shifted its focus towards practical applications for their HPS (High-Performance Sintering) 3D printing process. By harnessing the capabilities of HPS, Axtra3D aims to provide solutions that meet the demands of various industries, including aerospace, automotive, and healthcare. This expansion of practical applications reflects the increasing utilization and versatility of 3D printing technology.
Book and Product Recommendations
Book recommendation: “Introduction to Electronic Packaging: Unconventional Guide to Product Design”
For those interested in expanding their knowledge of electronic packaging and product design, “Introduction to Electronic Packaging: Unconventional Guide to Product Design” by S.A. Srinivasa Moorthy is a highly recommended read. This book provides valuable insights into the design principles and considerations for electronic packaging, offering a comprehensive guide for professionals and enthusiasts in the field.
Book recommendation: “Hit Makers: How to Succeed in an Age of Distraction”
In the age of information and constant distractions, “Hit Makers: How to Succeed in an Age of Distraction” by Derek Thompson provides valuable insights into the factors that contribute to the success of products, ideas, and individuals. This book explores the psychology behind what makes certain things popular and provides valuable lessons for professionals in various industries.
Ultimaker Cura’s secret lightning infill feature
Ultimaker Cura, one of the most popular 3D printing software programs, has a secret feature known as the lightning infill. This feature enables users to enhance the strength and speed of their prints by adjusting the infill pattern. By utilizing this hidden feature, users can optimize their prints and achieve superior results with their Ultimaker 3D printers.
Buy Photon Mono M5 Get Free 1KG Resin