ANYCUBIC 10K Resin 3D Printer, Photon Mono 4 LCD 3D Printer with 7-inch Mono Screen, Upgraded LighTurbo Matrix and Printing Platform, Printing Size of 6.04''x3.42''x6.49''
$169.99 (as of March 8, 2025 20:51 GMT +00:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)Have you ever wondered about the latest advancements in medical device technology, particularly in spinal implants? There’s some exciting news in this field that might pique your interest. The FDA has recently granted clearance to Nvision’s innovative 3D printed PEEK Interbody System. Let’s delve into the fascinating journey of this breakthrough, understand its benefits, and explore what it means for the future of spinal surgeries.
Buy Photon Mono M5 Get Free 1KG Resin
FDA Clears Nvision’s 3D Printed PEEK Interbody System
An Overview of PEEK and Its Significance
Polyether ether ketone (PEEK) is a high-performance polymer that’s been transforming implant technology. Known for its exceptional mechanical properties and biocompatibility, PEEK has been used in over 15 million implants. Its modulus of elasticity is close to that of human bone, which minimizes the risk of subsidence. Additionally, its radiolucency allows surgeons to monitor the fusion process post-surgery accurately.
The Role of Invibio Biomaterial Solutions
Invibio Biomaterial Solutions, a leader in high-performance biomaterial solutions, has developed PEEK-OPTIMA, a specific type of PEEK used in Nvision’s new system. With the groundbreaking Bond3D additive manufacturing technology, Invibio has pushed the boundaries of what’s possible in medical implant design. This technology enables the creation of intricate structures, which was previously unthinkable with conventional methods.
Understanding the Bond3D Technology
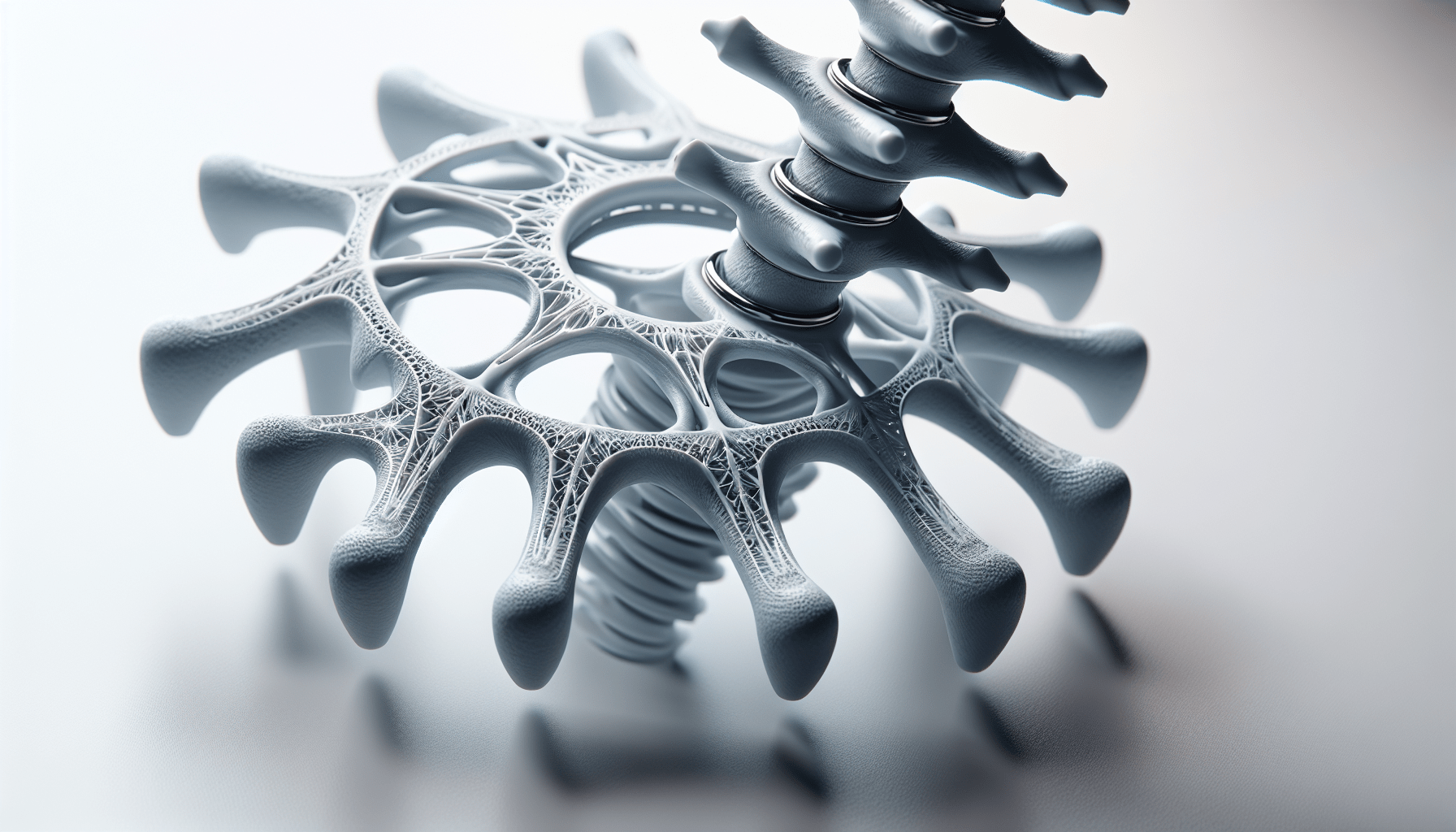
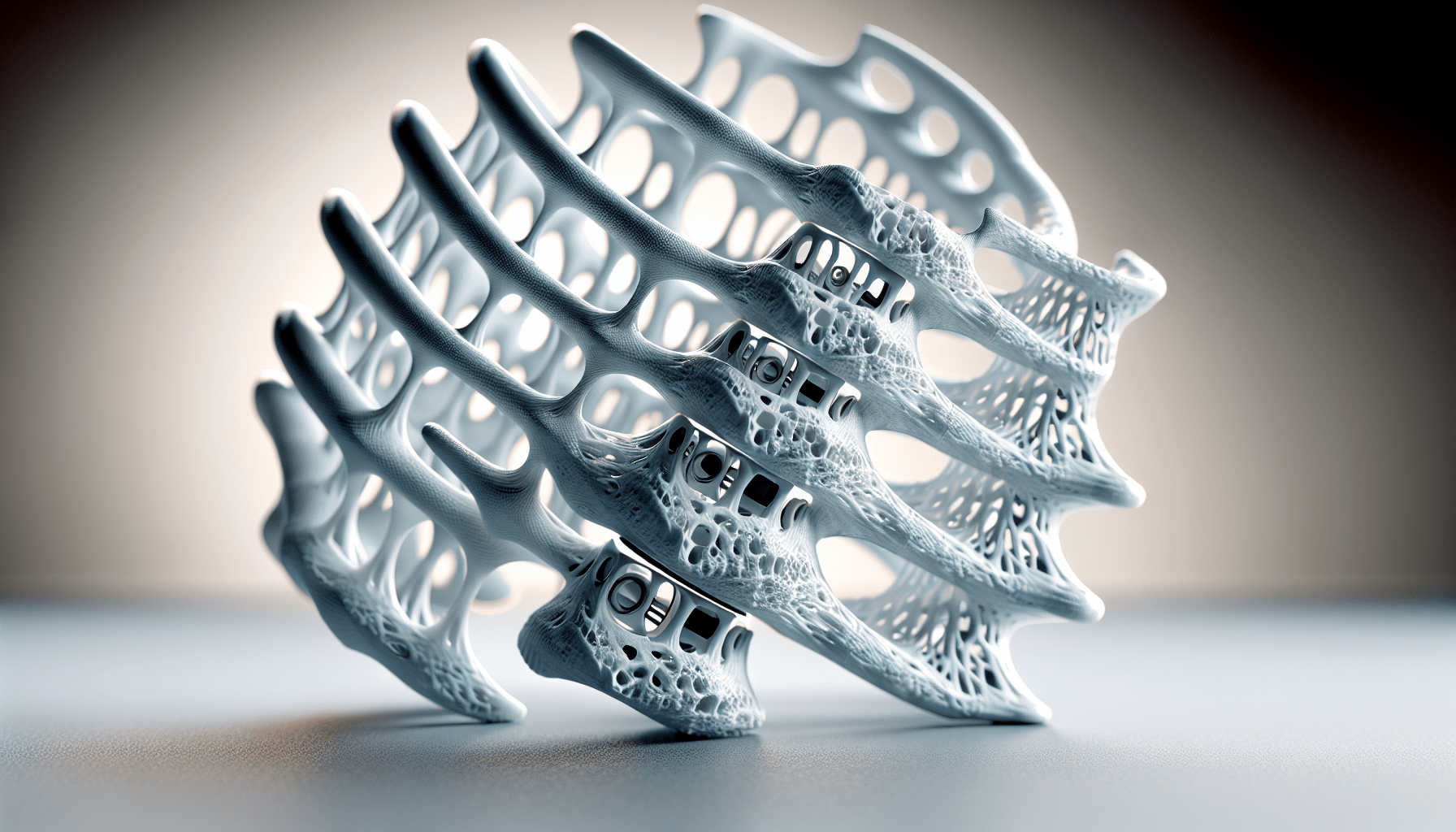
Bond3D additive manufacturing technology allows for the creation of solid and porous areas within the same implant. This design freedom is crucial because it enables the incorporation of porous structures that enhance bone ingrowth and device fixation. This means that the implants can meld with your existing bone more effectively, offering better stability and integration.
Nvision’s 3D Printed PEEK Interbody System: A Closer Look
What Makes It Innovative?
Nvision Biomedical Technologies, based in San Antonio, has co-developed this revolutionary system with Invibio. The 3D printed PEEK Interbody System comprises Cervical and Anterior Lumbar Interbody Fusion (ALIF) devices. These devices are unique because they integrate extensive porous structures, promoting multi-directional bone ingrowth. This innovation is the first of its kind to combine PEEK-OPTIMA with the design capabilities offered by Bond3D technology.
| Feature | Description |
|---|---|
| Material | PEEK-OPTIMA |
| Compatibility | Cervical and ALIF devices |
| Porous Structures | Multi-directional bone ingrowth |
| Imaging Capability | Superior to titanium implants |
| Modulus of Elasticity | Similar to human bone |
Advantages Over Conventional Implants
One of the primary advantages of PEEK-OPTIMA is its mechanical properties, which closely mimic human bone, reducing stress shielding and promoting natural bone growth. Additionally, PEEK-OPTIMA’s superior imaging capabilities allow for better monitoring of the fusion process compared to titanium implants, which can obscure the view on radiographic images.
Statements from Key Players
Brian Kieser, CEO of Nvision Biomedical Technologies, expressed his excitement, stating, “Our partnership with Invibio on this project showcases our commitment to pushing the boundaries of medical device innovation.” The sentiment is shared by Tom Zink, Senior Vice President of Product Development at Nvision, who believes that leveraging this advanced PEEK additive manufacturing platform allows for more innovative designs and addresses previous limitations.
$30 off $400+ Anycubic Products with code AC30OFF
Clinical Perspectives: What Surgeons Are Saying
The Clinical Benefits
Dr. Steven Lee, a spine and orthopedic surgeon, has highlighted several benefits of the new interbody devices. He points out that PEEK-OPTIMA’s modulus of elasticity helps prevent subsidence, while its radiolucency allows for the confirmation of the fusion process. The porous structure mimics native bone, encouraging bone growth into the device itself and thus enhancing the construct stability.
Improved Patient Outcomes
The combined characteristics of strength, reduced subsidence, boney ingrowth, and radiolucency offer significant clinical benefits. These attributes not only ensure that the implants are more secure but also enable better post-operative monitoring, leading to improved patient outcomes.
The Development Journey: From Concept to FDA Clearance
Partnership and Collaboration
The collaboration between Nvision and Invibio began with a shared vision of innovating spinal implants. Invibio played a crucial role in the development of PEEK-OPTIMA and the Bond3D technology platform. The rigorous process involved extensive performance testing and the filing of a new master file (MAF) with the FDA to support this and future regulatory submissions.
Regulatory Approval
Obtaining FDA clearance is a significant milestone. The thorough review process ensures that the new 3D printed PEEK Interbody System meets stringent safety and efficacy standards. This clearance paves the way for Nvision to introduce its groundbreaking products to the market, offering new solutions to surgeons and patients.
Future Prospects: What This Means for the Medical Device Industry
The Potential for Broader Applications
The proprietary Bond3D advanced manufacturing process used in this device is now available through Invibio to other medical device companies. This availability allows for greater design freedom and the realization of innovative concepts that were previously impossible with conventional manufacturing methods.
Nvision’s Expanding Portfolio
Brian Kieser indicates that this FDA clearance builds on a history of successful co-development between Nvision and Invibio. Notably, their collaborative efforts have extended to spine and foot-and-ankle devices. The new 3D printed PEEK Cervical and ALIF lines are available in various footprints and lordotic angles, all featuring porous design elements aimed at promoting bone ingrowth.
Industry Response
The medical community is enthusiastically embracing these advancements. The combination of solid and intricately porous PEEK-OPTIMA structures within the Nvision system provides potential for enhanced fixation and imaging benefits. John Devine, MD of Invibio, emphasizes that this capability represents a substantial breakthrough for device companies.
A Paradigm Shift in Spinal Surgery
Enhanced Stability and Bone Integration
The porous structures within the implants facilitate better bone integration, which is crucial for the long-term success of spinal surgeries. These structures allow bones to grow into the device, ensuring a more secure and stable integration.
Radiolucency for Better Monitoring
The radiolucent nature of PEEK-OPTIMA means that post-surgical monitoring is more effective. Surgeons can clearly see how the bones are fusing, making it easier to assess the success of the procedure and take timely action if needed.
Mechanical Properties That Match Bone
The mechanical properties of PEEK-OPTIMA are quite similar to human bone, which minimizes stress shielding. This ensures that the implant works in harmony with the body’s natural biomechanics, reducing complications and enhancing recovery.
Looking Ahead: Opportunities and Challenges
Technological Advancements
Advancements in additive manufacturing, like Bond3D technology, are paving the way for more customized and effective medical devices. The ability to create implants with varying densities and intricate designs opens up new possibilities for patient-specific solutions.
Regulatory and Market Considerations
While the FDA clearance is a significant achievement, navigating the regulatory landscape remains a challenge for new medical technologies. Continuous collaboration with regulatory bodies, thorough testing, and adherence to safety standards are essential for bringing such innovations to market.
Global Impact
As this technology gains acceptance and proves its efficacy, there’s potential for a global impact. Countries around the world could benefit from these advanced implants, improving healthcare outcomes on a broad scale.
Conclusion
The FDA’s clearance of Nvision’s 3D printed PEEK Interbody System marks a significant milestone in the field of medical implants. This innovative technology offers numerous benefits, from enhanced bone ingrowth to better imaging capabilities. The collaboration between Nvision and Invibio has resulted in a groundbreaking product that promises to improve patient outcomes and drive further advancements in medical device technology. As we look to the future, the potential applications and benefits of this technology are vast, heralding a new era in spinal surgery and beyond. So, the next time you hear about advancements in medical technology, you’ll understand the profound impact innovations like Nvision’s 3D printed PEEK Interbody System can have on healthcare and patient well-being.
Buy Photon Mono M5 Get Free 1KG Resin